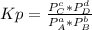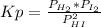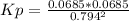Chemistry, 26.06.2020 15:01 wrightstephanie193
2. A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen and iodine:2HI (g) H2(g) + I2(g)When the system comes to equilibrium at 425 °C, PHI = 0.794 atm, and PH2 = PI2 = 0.0685 atm. The value of Kp at this temperature is .

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2

Chemistry, 23.06.2019 12:00
Which of the following statements is true? a. most heat energy is easily recovered and used for useful actions. b. friction causes molecules to vibrate more slowly. burning air and gasoline in an c. engine changes chemical energy into mechanical energy. it is impossible to d. change mechanical energy into mechanical energy.
Answers: 1

You know the right answer?
2. A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrog...
Questions


Arts, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

Health, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

English, 11.03.2021 19:00


Mathematics, 11.03.2021 19:00

Biology, 11.03.2021 19:00



Biology, 11.03.2021 19:00



Mathematics, 11.03.2021 19:00

Computers and Technology, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

Chemistry, 11.03.2021 19:00