Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
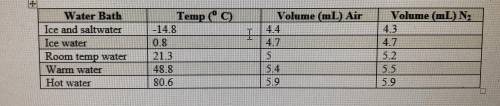
If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe...
Questions



History, 04.02.2021 21:30





Computers and Technology, 04.02.2021 21:30


Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30




History, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30

Mathematics, 04.02.2021 21:30


Physics, 04.02.2021 21:30