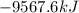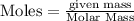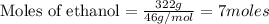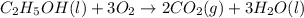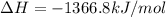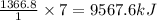Chemistry, 26.06.2020 15:01 nehaljay1883
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g)⟶2CO2(g)+3H2O(l)ΔH∘ c=−1366.8kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Calculate the change in enthalpy associated with the combustion of 322 g of ethanol. C2H5OH(l)+3O2(g...
Questions



Advanced Placement (AP), 14.12.2020 23:30

Biology, 14.12.2020 23:30






Mathematics, 14.12.2020 23:30



Chemistry, 14.12.2020 23:30

History, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30




History, 14.12.2020 23:30