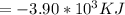Chemistry, 26.06.2020 15:01 coltonduggan
When 1.550 gg of liquid hexane (C6H14)(C6H14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87 ∘C∘C to 38.13 ∘C∘C. Find ΔErxnΔErxn for the reaction in kJ/molkJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kJ/∘CkJ/∘C.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
When 1.550 gg of liquid hexane (C6H14)(C6H14) undergoes combustion in a bomb calorimeter, the temper...
Questions





Chemistry, 26.04.2021 21:20

English, 26.04.2021 21:20

German, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Physics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20


Mathematics, 26.04.2021 21:20

Geography, 26.04.2021 21:20


Spanish, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20

Mathematics, 26.04.2021 21:20