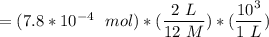Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
6. To isolate benzoic acid from a bicarbonate solution, it is acidified with concen- trated hydrochl...
Questions


History, 23.07.2019 04:00

Mathematics, 23.07.2019 04:00


Social Studies, 23.07.2019 04:00

Biology, 23.07.2019 04:00


Biology, 23.07.2019 04:00

History, 23.07.2019 04:00

History, 23.07.2019 04:00

Chemistry, 23.07.2019 04:00

Biology, 23.07.2019 04:00


History, 23.07.2019 04:00

History, 23.07.2019 04:00

History, 23.07.2019 04:00

Mathematics, 23.07.2019 04:00