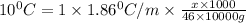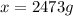Chemistry, 26.06.2020 16:01 FantasticFerret
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution that freezes at −10.0°C? Assume the density of water is 1.0 g/mL. Kf of water is 1.86°C/m.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
What mass of ethanol, C2H5OH a nonelectrolyte, must be added to 10.0 L of water to give a solution t...
Questions





Physics, 18.10.2019 10:10

Mathematics, 18.10.2019 10:10

Mathematics, 18.10.2019 10:10

History, 18.10.2019 10:10

Mathematics, 18.10.2019 10:10

Mathematics, 18.10.2019 10:10

Mathematics, 18.10.2019 10:10


English, 18.10.2019 10:10

Biology, 18.10.2019 10:10

Arts, 18.10.2019 10:10



Biology, 18.10.2019 10:10

Biology, 18.10.2019 10:10

History, 18.10.2019 10:10

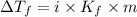
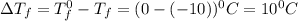 = Depression in freezing point
= Depression in freezing point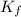 = freezing point constant for water=
= freezing point constant for water= 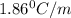
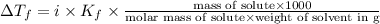
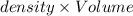
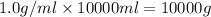 ( 1L=1000ml)
( 1L=1000ml)