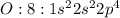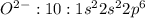Chemistry, 26.06.2020 16:01 HELPPPPPfawfds
13. Would you expect oxygen to form a cation or anion? How many electrons would it gain or lose? Why?
A)Anion, it would lose 2 electrons to satisfy the octet rule.
B)Cation, it would lose 2 electrons to satisfy the octet rule.
C)Anion, it would gain 2 electrons to satisfy the octet rule.
D)Cation, it would gain 2 electrons to satisfy the octet rule.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
13. Would you expect oxygen to form a cation or anion? How many electrons would it gain or lose? Why...
Questions

English, 13.12.2019 21:31



Mathematics, 13.12.2019 21:31


Mathematics, 13.12.2019 21:31






Biology, 13.12.2019 21:31

Mathematics, 13.12.2019 21:31


Mathematics, 13.12.2019 21:31

Mathematics, 13.12.2019 21:31

History, 13.12.2019 21:31


Chemistry, 13.12.2019 21:31

Social Studies, 13.12.2019 21:31