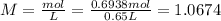Chemistry, 26.06.2020 17:01 villafana36
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16 g/mol, what is the molarity of this solution? answer options are 0.0106 M C6H12O6 0.0195 M C6H12O6 1.07 M C6H12O6 1.92 M C6H12O6
need help ASAP
will mark brainlest

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1
You know the right answer?
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16...
Questions

Social Studies, 29.01.2021 23:30



Mathematics, 29.01.2021 23:30

Mathematics, 29.01.2021 23:30



Mathematics, 29.01.2021 23:30



Mathematics, 29.01.2021 23:30

Mathematics, 29.01.2021 23:30

Medicine, 29.01.2021 23:30

Health, 29.01.2021 23:30





Mathematics, 29.01.2021 23:30

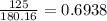 mol de glucosa
mol de glucosa