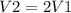Chemistry, 27.06.2020 06:01 whyidkmyself
Be sure to answer all parts. What is the effect of each of the following on the volume of 1 mol of an ideal gas? (a) The pressure changes from 760 torr to 202 kPa, and the temperature changes from 37°C to 155 K. The volume increases by a factor of 2. The volume increases by a factor of 4. The volume decreases by a factor of 2. The volume decreases by a factor of 4. The volume remains the same. (b) The temperature changes from 305 K to 32°C, and the pressure changes from 2 atm to 101 kPa. The volume increases by a factor of 2. The volume increases by a factor of 4. The volume decreases by a factor of 2. The volume decreases by a factor of 4. The volume remains the same.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Be sure to answer all parts. What is the effect of each of the following on the volume of 1 mol of a...
Questions



Mathematics, 13.09.2021 01:00



Chemistry, 13.09.2021 01:00


History, 13.09.2021 01:00


Mathematics, 13.09.2021 01:00


Biology, 13.09.2021 01:00



English, 13.09.2021 01:00


Mathematics, 13.09.2021 01:00

Chemistry, 13.09.2021 01:00


Mathematics, 13.09.2021 01:00