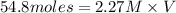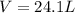Chemistry, 27.06.2020 20:01 jacobdobson5856
One of the first steps in the enrichment of uranium for use in nuclear power plants involves a displacement reaction between UO2 and aqueous HF: UO2(s) + HF(aq) → UF4(s) + H2O(l) [unbalanced] How many liters of 2.27 M HF will react with 3.70 kg of UO2?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
One of the first steps in the enrichment of uranium for use in nuclear power plants involves a displ...
Questions

History, 05.10.2019 15:00

Biology, 05.10.2019 15:00

Mathematics, 05.10.2019 15:00

Geography, 05.10.2019 15:00



English, 05.10.2019 15:00



Mathematics, 05.10.2019 15:00

Mathematics, 05.10.2019 15:00

Biology, 05.10.2019 15:00

Biology, 05.10.2019 15:00



Mathematics, 05.10.2019 15:00




Mathematics, 05.10.2019 15:00

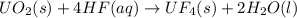
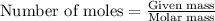
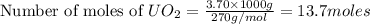
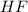
 of
of 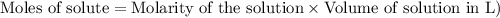 .....(1)
.....(1)