
Chemistry, 27.06.2020 02:01 jennymares
Consider the equation below. CaCO3(S) CaO(S) + CO2(g) What is the equilibrium constant expression for the given reaction?
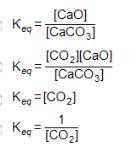
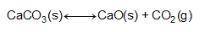

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Consider the equation below. CaCO3(S) CaO(S) + CO2(g) What is the equilibrium constant expression fo...
Questions

Computers and Technology, 27.02.2020 21:12







English, 27.02.2020 21:12









Spanish, 27.02.2020 21:14

Mathematics, 27.02.2020 21:14




