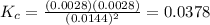Chemistry, 27.06.2020 15:01 cynthiafchs9203
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibrium concentration of H2 is 0.0028M, then what is the equilibrium constant of the reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
Given the reaction: 2HF(g) H2(g) F2(g) If the initial concentration of HF is 0.025M and the equilibr...
Questions










Mathematics, 26.03.2020 20:39

Mathematics, 26.03.2020 20:39




Social Studies, 26.03.2020 20:39


Mathematics, 26.03.2020 20:39

History, 26.03.2020 20:39


Chemistry, 26.03.2020 20:39

![K_{c} =\frac{[H_{2}][F_{2}] }{[HF]^2}](/tpl/images/0695/8873/96c9f.png)