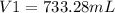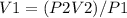Chemistry, 27.06.2020 17:01 wolfking800
A sample of chlorine gas starting at 686 mm Hg is placed under a pressure of 991 mm Hg and reduced to a volume of 507.6 mL. What was the initial volume of the chlorine gas container if the process was performed at constant temperature

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
A sample of chlorine gas starting at 686 mm Hg is placed under a pressure of 991 mm Hg and reduced t...
Questions

Mathematics, 19.06.2021 09:50


Mathematics, 19.06.2021 09:50

English, 19.06.2021 09:50

Mathematics, 19.06.2021 09:50

English, 19.06.2021 09:50

Mathematics, 19.06.2021 09:50

Mathematics, 19.06.2021 09:50

History, 19.06.2021 09:50

Mathematics, 19.06.2021 09:50


Social Studies, 19.06.2021 09:50

English, 19.06.2021 09:50


Health, 19.06.2021 09:50

English, 19.06.2021 09:50




Mathematics, 19.06.2021 09:50