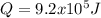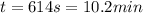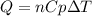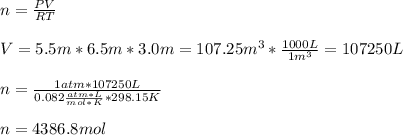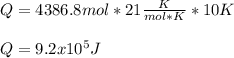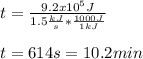The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. This is one of the reasons why desert region, although very hot during the day, are bitterly cold at night. The heat capacity of air at room temperature and pressure is appoximately 21 J/K*mol. How much energy is required to raise the temperature of a room of dimensions 5.5m x 6.5m x 3.0m by 10 degrees Celsius? If losses are neglected, how long will it take a heater rated at 1.5 kW to achieve that increase given that 1 W = 1 J/s?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2
You know the right answer?
The heat capacity of air is much smaller than that of water, and relatively modest amounts of heat a...
Questions

Social Studies, 28.01.2020 17:55

Mathematics, 28.01.2020 17:55

Advanced Placement (AP), 28.01.2020 17:55

Mathematics, 28.01.2020 17:55




Mathematics, 28.01.2020 17:55



Health, 28.01.2020 17:55



History, 28.01.2020 17:55

English, 28.01.2020 17:55

Mathematics, 28.01.2020 17:55

English, 28.01.2020 17:55

History, 28.01.2020 17:55

Computers and Technology, 28.01.2020 17:55