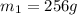Chemistry, 30.06.2020 18:01 6710000831
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 28.0∘C. What is the mass of the silver block?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated...
Questions



Biology, 22.01.2020 18:31



Mathematics, 22.01.2020 18:31





Mathematics, 22.01.2020 18:31

Mathematics, 22.01.2020 18:31



Spanish, 22.01.2020 18:31

Chemistry, 22.01.2020 18:31


Mathematics, 22.01.2020 18:31


Arts, 22.01.2020 18:31

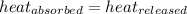
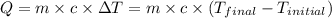
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0697/4196/09236.png) .................(1)
.................(1)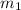 = mass of silver = ?
= mass of silver = ?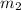 = mass of water = 100.0 g
= mass of water = 100.0 g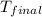 = final temperature =
= final temperature = 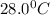
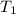 = temperature of silver =
= temperature of silver = 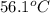
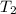 = temperature of water =
= temperature of water = 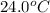
 = specific heat of silver =
= specific heat of silver = 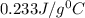
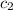 = specific heat of water=
= specific heat of water= 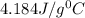
![-m_1\times 0.233\times (28.0-56.1)=[100.0\times 4.184\times (28.0-24.0)]](/tpl/images/0697/4196/c0833.png)