Chemistry, 29.06.2020 20:01 mdaniella522
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibrium. The equilibrium pressure of HI is found to be 1.738 atm. Calculate Kp for the reaction at this temperature. H2(g) + I2(g) <=> 2 HI(g). Give your answer to 3 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibri...
Questions

Mathematics, 26.09.2019 20:40

Chemistry, 26.09.2019 20:40


Mathematics, 26.09.2019 20:40

Mathematics, 26.09.2019 20:40

Arts, 26.09.2019 20:40







Mathematics, 26.09.2019 20:40



Biology, 26.09.2019 20:40

History, 26.09.2019 20:40

Social Studies, 26.09.2019 20:40


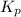 . To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.
. To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.![K_{p} =\frac{[HI]^2}{[H_{2}][I_{2} ] }](/tpl/images/0696/9799/d09a4.png)