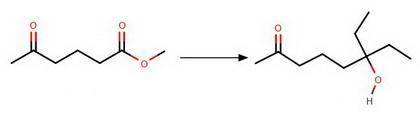
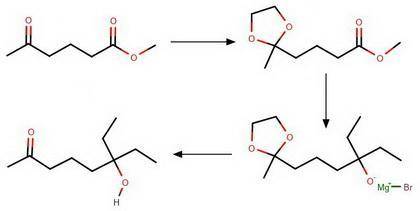
Chemistry, 01.07.2020 16:01 theyfallfora
Provide the reagents necessary to carry out the following conversion.
1. HOCH2CH2OH/H2SO4
2. LiAlH4
3. CH3CH2MgBr (2 eq)
1. H2SO4
2. CH3CH2OH
3. H3O
1. HOCH2CH2OH/H2SO4
2. CH3CH2MgBr (2 eq)
3. H3O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Provide the reagents necessary to carry out the following conversion.
1. HOCH2CH2OH/H2SO4
2....
2....
Questions




Physics, 05.03.2021 23:30


Mathematics, 05.03.2021 23:30



Mathematics, 05.03.2021 23:30



Mathematics, 05.03.2021 23:30


History, 05.03.2021 23:30

Mathematics, 05.03.2021 23:30

Mathematics, 05.03.2021 23:30


Mathematics, 05.03.2021 23:30

Mathematics, 05.03.2021 23:30

Social Studies, 05.03.2021 23:30