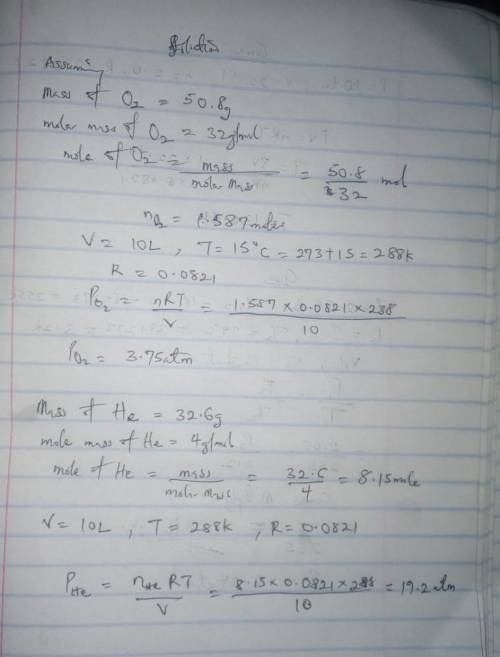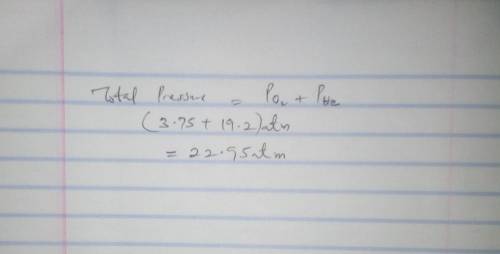Chemistry, 01.07.2020 16:01 rebekahwirogo
Calculate the partial pressure of each gas and the total pressure if the temperature of the gas is 15 ∘C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Calculate the partial pressure of each gas and the total pressure if the temperature of the gas is 1...
Questions

Mathematics, 11.01.2021 17:40

French, 11.01.2021 17:40

Social Studies, 11.01.2021 17:40






Social Studies, 11.01.2021 17:40

Social Studies, 11.01.2021 17:40



Mathematics, 11.01.2021 17:40


Mathematics, 11.01.2021 17:40



English, 11.01.2021 17:40


Spanish, 11.01.2021 17:40