pick the atom with

Chemistry, 30.06.2020 18:01 thutch1950oww9q0
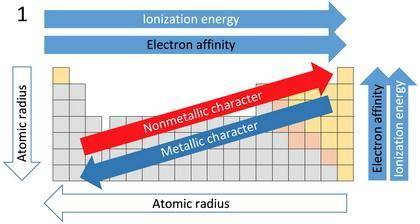
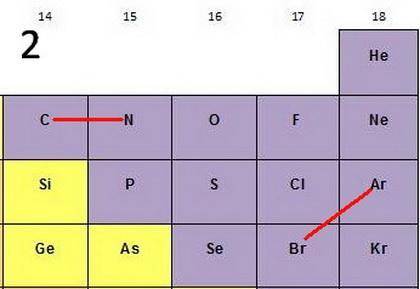
For each of the following pairs of elements
(1C and N2) (1Ar and Br2)
pick the atom with
a. more favorable (exothermic) electron affinity.
b. higher ionization energy.
c. larger size.
How do you even go about do this?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
For each of the following pairs of elements
(1C and N2) (1Ar and Br2)
pick the atom with
pick the atom with
Questions

History, 17.01.2020 17:31




Mathematics, 17.01.2020 17:31


Mathematics, 17.01.2020 17:31











History, 17.01.2020 17:31