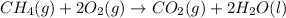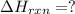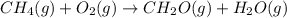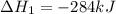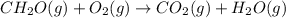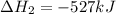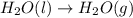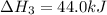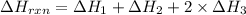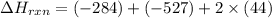Chemistry, 01.07.2020 22:01 catsRlife4573
Calculate ΔHrxnΔHrxn for the following reaction: CH4(g)+2O2(g)→CO2(g)+2H2O(l)CH4(g)+ 2O2(g)→CO2(g)+2H2O(l) Use the following reactions and given ΔHΔH values. CH4(g)+O2(g)→CH2O(g)+H2O(g)CH4(g)+O 2(g)→CH2O(g)+H2O(g), ΔH=−ΔH=−284 kJkJ CH2O(g)+O2(g)→CO2(g)+H2O(g)CH2O(g)+ O2(g)→CO2(g)+H2O(g), ΔH=−ΔH=−527 kJkJ H2O(l)→H2O(g)H2O(l)→H2O(g), ΔH=ΔH= 44.0 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3
You know the right answer?
Calculate ΔHrxnΔHrxn for the following reaction: CH4(g)+2O2(g)→CO2(g)+2H2O(l)CH4(g)+ 2O2(g)→CO2(g)+2...
Questions



Mathematics, 04.10.2021 07:00

Mathematics, 04.10.2021 07:00

Mathematics, 04.10.2021 07:00

Geography, 04.10.2021 07:00

Business, 04.10.2021 07:00


Mathematics, 04.10.2021 07:00


History, 04.10.2021 07:10

History, 04.10.2021 07:10

Mathematics, 04.10.2021 07:10

Mathematics, 04.10.2021 07:10

Mathematics, 04.10.2021 07:10




English, 04.10.2021 07:10