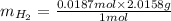Chemistry, 01.07.2020 16:01 kierraivey
find the mass of h2 produced Binary compounds of alkali metals and hydrogen react with water to produce H2(g). The H2H2 from the reaction of a sample of NaH with an excess of water fills a volume of 0.505 L above the water. The temperature of the gas is 35 ∘C∘C and the total pressure is 755 mmHg

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
find the mass of h2 produced Binary compounds of alkali metals and hydrogen react with water to prod...
Questions




English, 03.03.2020 21:30



Biology, 03.03.2020 21:30

Mathematics, 03.03.2020 21:30





Mathematics, 03.03.2020 21:30

English, 03.03.2020 21:31






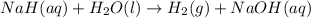
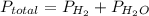
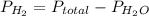
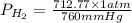
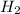 as follows.
as follows.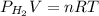
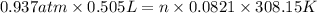
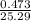 mol
mol