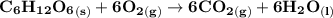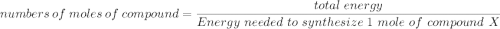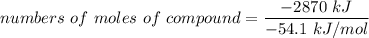Chemistry, 02.07.2020 22:01 treypickich14
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and water liberates 2870 kJ2870 kJ of energy (ΔG°′=−2870 kJ/mol(ΔG°′=−2870 kJ/mol ). If the energy generated by the combustion of glucose is entirely converted to the synthesis of a hypothetical compound X, calculate the number of moles of the compound that could theoretically be generated. Use the value ΔG°′compound X=−54.1 kJ/molΔG°′compound X=−54.1 kJ/mol . Round your answer to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and...
Questions

Mathematics, 05.05.2020 17:07

Mathematics, 05.05.2020 17:07



Social Studies, 05.05.2020 17:07

Social Studies, 05.05.2020 17:07


Mathematics, 05.05.2020 17:07




Mathematics, 05.05.2020 17:07



Health, 05.05.2020 17:07

Spanish, 05.05.2020 17:07


Mathematics, 05.05.2020 17:07


Mathematics, 05.05.2020 17:07

 53 mole
53 mole