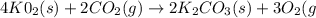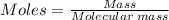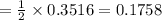Chemistry, 02.07.2020 15:01 MalikaJones
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PPE) for first responders. SCBA provide breathing oxygen in a contained system for use in low-oxygen environments or in the presence of toxic fumes. The oxygen is generated through the reaction of potassium superoxide, KO2 and carbon dioxide, forming potassium carbonate and oxygen gas. If the 25.0 g KO2 in the SCBA system was exposed to 45.0 g CO2, what scenario best describes the outcome:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0 c?
Answers: 1
You know the right answer?
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PP...
Questions

Biology, 23.03.2021 14:40


History, 23.03.2021 14:40

Mathematics, 23.03.2021 14:40


Mathematics, 23.03.2021 14:40

English, 23.03.2021 14:40


Mathematics, 23.03.2021 14:40



Mathematics, 23.03.2021 14:50

Chemistry, 23.03.2021 14:50

English, 23.03.2021 14:50

Social Studies, 23.03.2021 14:50


Chemistry, 23.03.2021 14:50

Mathematics, 23.03.2021 14:50