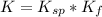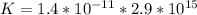Chemistry, 02.07.2020 16:01 jalynholden07
Consider the insoluble compound zinc carbonate , ZnCO3 . The zinc ion also forms a complex with hydroxide ions . Write a balanced net ionic equation to show why the solubility of ZnCO3 (s) increases in the presence of hydroxide ions and calculate the equilibrium constant for this reaction. For Zn(OH)42- , Kf = 2.9×1015 . Use the pull-down boxes to specify states such as (aq) or (s).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Consider the insoluble compound zinc carbonate , ZnCO3 . The zinc ion also forms a complex with hydr...
Questions


Computers and Technology, 07.10.2021 03:20

English, 07.10.2021 03:20

Mathematics, 07.10.2021 03:20



Computers and Technology, 07.10.2021 03:20

Computers and Technology, 07.10.2021 03:20

History, 07.10.2021 03:20

Physics, 07.10.2021 03:20


English, 07.10.2021 03:20

Social Studies, 07.10.2021 03:20

World Languages, 07.10.2021 03:20

Mathematics, 07.10.2021 03:20



Mathematics, 07.10.2021 03:20

Arts, 07.10.2021 03:20

Business, 07.10.2021 03:30

![ZnCO_3 _{(s)} + 4 OH^{-}_{(aq)} \to [Zn(OH)_4]^{2-} _{(aq)} + CO_3^{2-} _{(aq)}](/tpl/images/0699/8948/a1741.png)
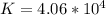
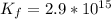
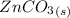 ⇔
⇔ 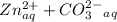 The solubility product constant for stage is
The solubility product constant for stage is 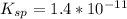
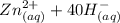 ⇔
⇔ ![[Zn(OH_4)]^{2-} _{(aq)}](/tpl/images/0699/8948/a852d.png) The formation constant for this step is given as
The formation constant for this step is given as