Chemistry, 03.07.2020 20:01 kwoolfe59006
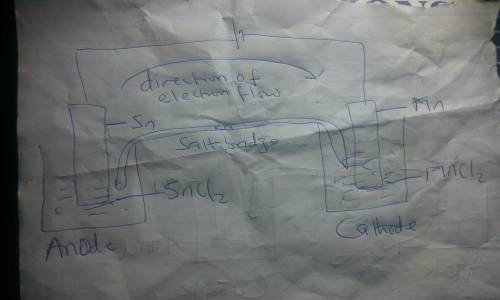
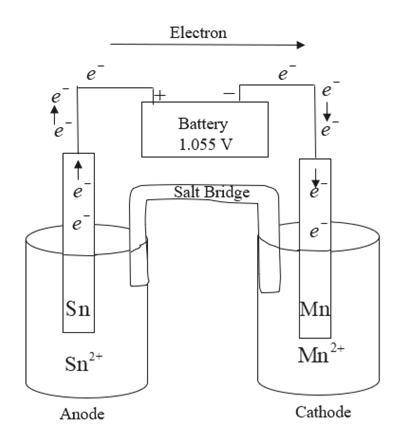
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standard conditions).
Part A Label the anode and cathode, indicate the direction of electron flow.
Drag the appropriate labels to their respective targets.
Part B Write an equation for the half-reaction occurring at each electrode.
Express your answers as chemical equations separated by a comma. Identify all of the phases in your answer.
Part C What minimum voltage is necessary to drive the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1


Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standar...
Questions


Advanced Placement (AP), 04.07.2019 03:30

English, 04.07.2019 03:30



English, 04.07.2019 03:30


History, 04.07.2019 03:30


History, 04.07.2019 03:30

Mathematics, 04.07.2019 03:30


Geography, 04.07.2019 03:30



Computers and Technology, 04.07.2019 03:30

Mathematics, 04.07.2019 03:30

World Languages, 04.07.2019 03:30