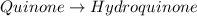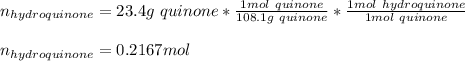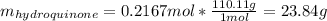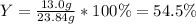Chemistry, 04.07.2020 01:01 allieb12334
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment. The reactant, quinone, is the limiting reagent. (To avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.)
Reactant mass 23.4g
Product mass 13.0g
Reactant moles 0.2167 mol
Reactant mass 23.4g
Product mass 13.0g
Molar mass C 12.0 g/mol
Molar mass H 1.00 g/mol
Molar mass O 16.0 g/mol
Theoretical maximum moles of hydroquinone:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Determine the theoretical maximum moles of hydroquinone, , that could be produced in this experiment...
Questions

Arts, 30.11.2020 22:10

Biology, 30.11.2020 22:10


Mathematics, 30.11.2020 22:10






Mathematics, 30.11.2020 22:10



History, 30.11.2020 22:10


English, 30.11.2020 22:10

History, 30.11.2020 22:10


Mathematics, 30.11.2020 22:10