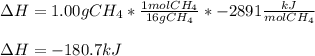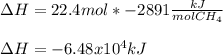Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enth...

Chemistry, 04.07.2020 02:01 khikhi1705
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enthalpy change for each of the following cases:
a. 1.00 g methane is burned in excess oxygen.
b. 1.00 3 10^3 L methane gas at 740. torr and 258°C are burned in excess oxygen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Questions



Mathematics, 24.08.2019 23:30

Geography, 24.08.2019 23:30




Biology, 24.08.2019 23:30

Biology, 24.08.2019 23:30



Geography, 24.08.2019 23:30



Mathematics, 24.08.2019 23:30

Geography, 24.08.2019 23:30

Chemistry, 24.08.2019 23:30

Mathematics, 24.08.2019 23:30

Social Studies, 24.08.2019 23:30