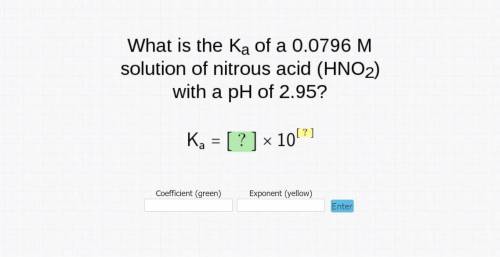
What is the Ka of a 0.0796 M solution of nitrous acid (HNO2) with a pH of 2.95?
...

Chemistry, 04.07.2020 04:01 quetzaliescalona
What is the Ka of a 0.0796 M solution of nitrous acid (HNO2) with a pH of 2.95?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Questions

Computers and Technology, 22.07.2019 20:40

Chemistry, 22.07.2019 20:40



Biology, 22.07.2019 20:40

Social Studies, 22.07.2019 20:40




Mathematics, 22.07.2019 20:40





Health, 22.07.2019 20:40



Social Studies, 22.07.2019 20:40

Spanish, 22.07.2019 20:40



