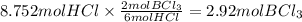Question 1
1 pts
2B+6HCI --
| --> 2BCl3 + 3H2
How many moles of boron chloride...


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Questions


Mathematics, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00


Mathematics, 09.07.2019 03:00


Biology, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00


Mathematics, 09.07.2019 03:00


Mathematics, 09.07.2019 03:00



Mathematics, 09.07.2019 03:00

Biology, 09.07.2019 03:00

Biology, 09.07.2019 03:00



Mathematics, 09.07.2019 03:00