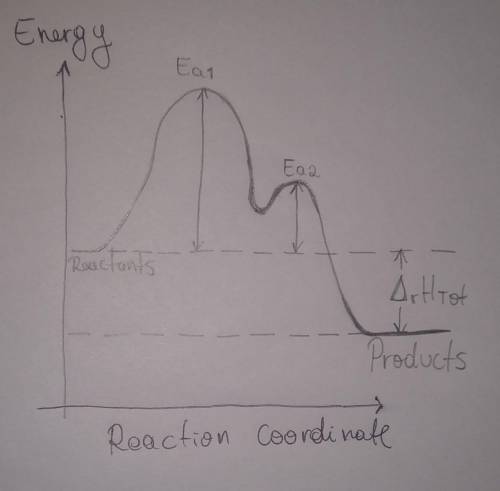
A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g) → NO (g) + NO3 (g)
Step 2: Fast, exothermic:
NO3 (g) + CO (g) → NO2 (g) + CO2 (g)
Overall reaction, exothermic:
NO2 (g) + CO (g) → NO (g) + CO2 (g)
a. Identify each of the following as a reactant, product, or intermediate:
1. NO2(g)
2. CO(g)
3. NO3(g)
4. CO2(g)
5. NO(g)
b. Draw a reaction coordinate diagram for this reaction. Indicate on this drawing the activation energy for each step and the overall enthalpy change.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
A proposed mechanism for the reaction of NO2 and CO is
Step 1: Slow, endothermic:
2 NO2 (g)...
2 NO2 (g)...
Questions




Engineering, 26.07.2019 18:20




History, 26.07.2019 18:20


Mathematics, 26.07.2019 18:20


Spanish, 26.07.2019 18:20







Computers and Technology, 26.07.2019 18:20

Computers and Technology, 26.07.2019 18:20