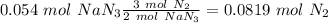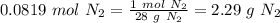Chemistry, 04.07.2020 14:01 Jakethedog210
Automotive air bags inflate when sodium azide, NaN3, decomposes explosively to its constituent elements. How many moles of nitrogen gas are produced by the decomposition of 3.55 mol of sodium azide?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Automotive air bags inflate when sodium azide, NaN3, decomposes explosively to its constituent eleme...
Questions

Mathematics, 06.05.2020 04:29

Mathematics, 06.05.2020 04:29

Biology, 06.05.2020 04:29

Mathematics, 06.05.2020 04:29

Mathematics, 06.05.2020 04:29


Mathematics, 06.05.2020 04:29








English, 06.05.2020 04:29

Chemistry, 06.05.2020 04:29

Mathematics, 06.05.2020 04:29

Mathematics, 06.05.2020 04:29


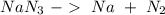
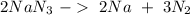
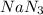 (65 g/mol), so:
(65 g/mol), so: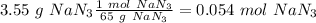
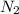 we can calculate the moles of
we can calculate the moles of