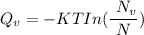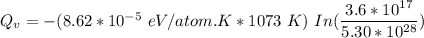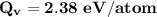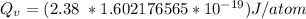Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1


You know the right answer?
Calculate the energy (in J/atom) for vacancy formation in silver, given that the equilibrium number...
Questions

Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

Computers and Technology, 25.05.2021 21:40


Social Studies, 25.05.2021 21:40


Mathematics, 25.05.2021 21:40



Mathematics, 25.05.2021 21:40



Mathematics, 25.05.2021 21:40


Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

Mathematics, 25.05.2021 21:40

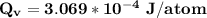
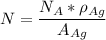
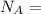 avogadro's number =
avogadro's number = 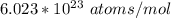
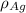 = Density of silver = 9.5 g/cm³
= Density of silver = 9.5 g/cm³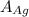 = Atomic weight of sliver = 107.9 g/mol
= Atomic weight of sliver = 107.9 g/mol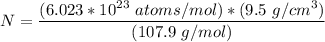
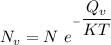
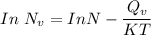
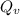 the subject of the formula; we have:
the subject of the formula; we have: