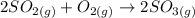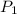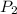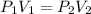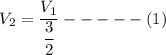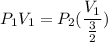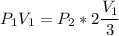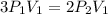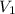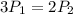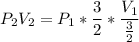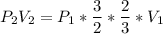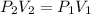Chemistry, 05.07.2020 14:01 jakobrobinette
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2
You know the right answer?
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) → 2SO3(g) Suppose th...
Questions

English, 25.11.2020 16:40

Spanish, 25.11.2020 16:40




Mathematics, 25.11.2020 16:40

English, 25.11.2020 16:40

Computers and Technology, 25.11.2020 16:40

Mathematics, 25.11.2020 16:40

English, 25.11.2020 16:40






Physics, 25.11.2020 16:40


Mathematics, 25.11.2020 16:40

Mathematics, 25.11.2020 16:40

Social Studies, 25.11.2020 16:50