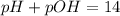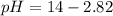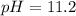Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
A chemist dissolves 867. mg of pure barium hydroxide in enough water to make up 170. mL of solution....
Questions


Mathematics, 02.10.2019 00:30



Computers and Technology, 02.10.2019 00:30

Mathematics, 02.10.2019 00:30


Mathematics, 02.10.2019 00:30




History, 02.10.2019 00:30


Business, 02.10.2019 00:30


Physics, 02.10.2019 00:30

History, 02.10.2019 00:30


English, 02.10.2019 00:30

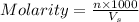
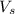 = volume of solution in ml
= volume of solution in ml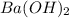 =
= 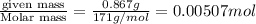 (1g=1000mg)
(1g=1000mg)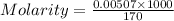
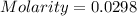
![pOH=-\log [OH^-]](/tpl/images/0701/6467/1fac1.png)
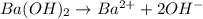
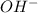
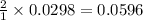 moles of
moles of ![pOH=-\log[0.0596]=2.82](/tpl/images/0701/6467/015aa.png)