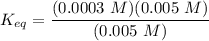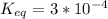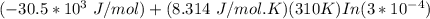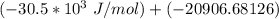Chemistry, 05.07.2020 14:01 lilrariwmb23701
A critical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, ATP, to adenosine diphosphate, ADP, as described by the reactionATP(aq)+ H2O(l) → ADP(aq)+ HPO4^-2 (aq)for which ΔGrxn = -30.5 kj/mol at 37.0C and pH 7.0. Required:a. Calculate the value of ΔGrxn in a biological cell in which [ATP] = 5.0 mM, [ADP] = 0.30 mM, and HPO4^-2= 5.0mMb. Is the hydrolysis of ATP spontaneous under these conditions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
A critical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions


Mathematics, 14.10.2019 12:10

Biology, 14.10.2019 12:10


Mathematics, 14.10.2019 12:10


Social Studies, 14.10.2019 12:10

Mathematics, 14.10.2019 12:10

Social Studies, 14.10.2019 12:10

Biology, 14.10.2019 12:10

Mathematics, 14.10.2019 12:10



English, 14.10.2019 12:10

Biology, 14.10.2019 12:10


Mathematics, 14.10.2019 12:10



Mathematics, 14.10.2019 12:10

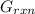 = −51. 4 kJ/mol
= −51. 4 kJ/mol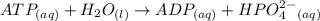
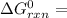 -30.5 kJ/mol
-30.5 kJ/mol![[HPO_4^{2-}}]](/tpl/images/0701/7595/d50c3.png) = 5.0 mM
= 5.0 mM can be expressed as:
can be expressed as:![K_{eq} = \dfrac{[ADP][ HPO_4^{2-}]} {[ATP]}](/tpl/images/0701/7595/e3711.png)