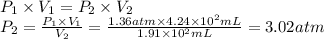Chemistry, 06.07.2020 17:01 lyssamichelle
A sample of oxygen, at 25°C, occupies a volume of 4.24 × 102 milliliters (mL) at 1.36 atm pressure. What pressure must be applied to compress the gas to a volume of 1.91 × 102 mL, with no temperature change?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3


Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1
You know the right answer?
A sample of oxygen, at 25°C, occupies a volume of 4.24 × 102 milliliters (mL) at 1.36 atm pressure....
Questions






Mathematics, 03.09.2020 04:01


Mathematics, 03.09.2020 04:01







History, 03.09.2020 04:01

Chemistry, 03.09.2020 04:01


Chemistry, 03.09.2020 04:01


Mathematics, 03.09.2020 04:01