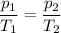Chemistry, 08.07.2020 01:01 jilliandantuma84
Determine the new temperature of a sample of gas if the pressure changes from 0.500 atm to 0.750 atm, and the gas had an initial temperature of 315 K. The volume and number of gas particles is held constant.
210. K
118 K
2.12 x 10-3 K
473 K

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
Determine the new temperature of a sample of gas if the pressure changes from 0.500 atm to 0.750 atm...
Questions


English, 20.05.2021 08:00



Mathematics, 20.05.2021 08:00

English, 20.05.2021 08:00



History, 20.05.2021 08:00

Mathematics, 20.05.2021 08:00

Mathematics, 20.05.2021 08:00

Physics, 20.05.2021 08:00

Business, 20.05.2021 08:00

Computers and Technology, 20.05.2021 08:00

Chemistry, 20.05.2021 08:00

Mathematics, 20.05.2021 08:00


Mathematics, 20.05.2021 08:00

Spanish, 20.05.2021 08:00

Mathematics, 20.05.2021 08:00