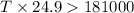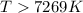For the reaction
N2(g) + O2(g)→2 NO(g) H° = 181 kJ and S° = 24.9 J/K G°
The equilibriu...

Chemistry, 08.07.2020 02:01 ordonez9029
For the reaction
N2(g) + O2(g)→2 NO(g) H° = 181 kJ and S° = 24.9 J/K G°
The equilibrium constant, K, would be greater than 1 at temperatures (above or below).. Kelvin.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Questions

Biology, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20



Health, 18.11.2020 01:20

Social Studies, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

Geography, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

Biology, 18.11.2020 01:20





Mathematics, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

Mathematics, 18.11.2020 01:20

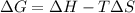
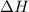 = enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J)
= enthalpy change = 181 kJ/mol = 181000 J/mol (1kJ=1000J)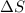 = entropy change= 24.9 J/Kmol
= entropy change= 24.9 J/Kmol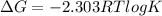
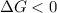
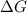 < 0,
< 0,  >
>