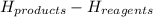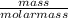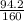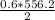A chemist measures the energy change Delta H during the following
2Fe2O3(s)->4FeO(s)+O2(g).
1) this reactions is: Endothermic or exothermic.
2) suppose 94.2g of Fe2O3 react. will any heat be relased or absorbed. yes absorbed. yes releases. no.
3) If you said heat will be released or absorbed in the second part of the question. calculate how much heat will be absored or released. be sure your answer has correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2


Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
A chemist measures the energy change Delta H during the following
2Fe2O3(s)->4FeO(s)+O2(g).
Questions

Biology, 20.11.2021 14:00

English, 20.11.2021 14:00


Social Studies, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00



Chemistry, 20.11.2021 14:10

Health, 20.11.2021 14:10

Mathematics, 20.11.2021 14:10

SAT, 20.11.2021 14:10

Mathematics, 20.11.2021 14:10

Mathematics, 20.11.2021 14:10



Mathematics, 20.11.2021 14:10


Mathematics, 20.11.2021 14:10