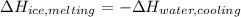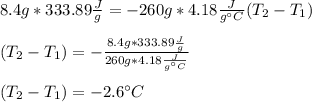An 8.4 g ice cube is placed into 260 g of water. Calculate the temperature change in the water upon the complete melting of the ice. Assume that all of the energy required to melt the ice comes from the water. Express your answer in terms of the initial temperature of water, T. a. -0.033T - 2.6 °Cb. -2.6T + 0.033 °Cc. 0.033T - 2.6 °Cd. 2.6T -0.033 °C

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
An 8.4 g ice cube is placed into 260 g of water. Calculate the temperature change in the water upon...
Questions


Mathematics, 16.04.2020 01:45

Mathematics, 16.04.2020 01:45

Mathematics, 16.04.2020 01:45

English, 16.04.2020 01:45


Mathematics, 16.04.2020 01:45

Health, 16.04.2020 01:45





Mathematics, 16.04.2020 01:45



Mathematics, 16.04.2020 01:45


Mathematics, 16.04.2020 01:45

Mathematics, 16.04.2020 01:46