
Chemistry, 07.07.2020 19:01 JayLiz1737
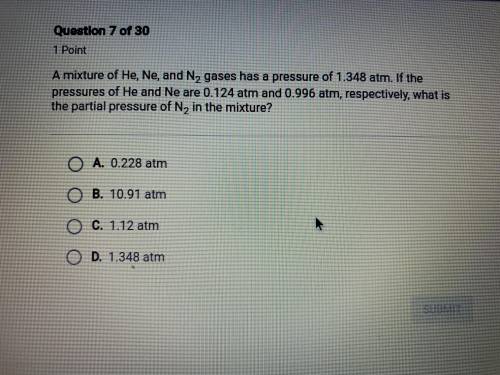
hurry please! a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.124 atm and 0.996 atm, respectively, what is the partial pressure of N2 in the mixture?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
hurry please! a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He...
Questions












Biology, 13.10.2020 03:01





Computers and Technology, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01

Mathematics, 13.10.2020 03:01




