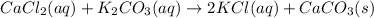Chemistry, 08.07.2020 22:01 Cutiepie55561
Aqueous calcium chloride reacts with aqueous potassium carbonate in a double-displacement reaction. Write a balanced equation to describe this reaction. Include states of matter in your answer. Click in the answer box to open the symbol palett

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Aqueous calcium chloride reacts with aqueous potassium carbonate in a double-displacement reaction....
Questions


Mathematics, 18.04.2020 04:19


English, 18.04.2020 04:19

Spanish, 18.04.2020 04:30



Mathematics, 18.04.2020 04:30




Biology, 18.04.2020 04:30








Mathematics, 18.04.2020 04:30