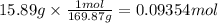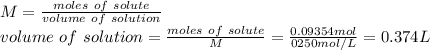A student wants to make a 0.250 M aqueous solution of silver nitrate, AgNO3, and has a bottle containing 15.89 g of silver nitrate. What should be the final volume of the solution? When you give your numerical answer, what is the correct significant figures and how do you know that is the correct amount?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
A student wants to make a 0.250 M aqueous solution of silver nitrate, AgNO3, and has a bottle contai...
Questions


English, 26.09.2019 11:00

Mathematics, 26.09.2019 11:00



English, 26.09.2019 11:00

Social Studies, 26.09.2019 11:00

History, 26.09.2019 11:00

Mathematics, 26.09.2019 11:00



History, 26.09.2019 11:00





History, 26.09.2019 11:10



Biology, 26.09.2019 11:10