
Chemistry, 08.07.2020 14:01 katier9407
It takes to break an carbon-chlorine single bond. Calculate the maximum wavelength of light for which an carbon-chlorine single bond could be broken by absorbing a single photon. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
It takes to break an carbon-chlorine single bond. Calculate the maximum wavelength of light for whic...
Questions


Computers and Technology, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50


Mathematics, 23.11.2020 19:50

World Languages, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50




English, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50


Business, 23.11.2020 19:50

History, 23.11.2020 19:50


Computers and Technology, 23.11.2020 19:50

Mathematics, 23.11.2020 19:50

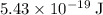 of energy to break one
of energy to break one  single bond.
single bond. 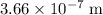 (in vacuum.) That's the same as
(in vacuum.) That's the same as 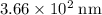 (rounded to three significant figures.)
(rounded to three significant figures.)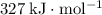 (note that the exact value can varies across sources.) In other words, it would take approximately
(note that the exact value can varies across sources.) In other words, it would take approximately 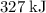 of energy to break one mole of these bonds.
of energy to break one mole of these bonds. 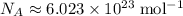 gives the number of
gives the number of  .
.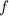 of a photon to its energy
of a photon to its energy 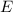 :
: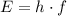 .
.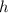 here represents the Planck Constant:
here represents the Planck Constant: 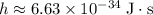 .
.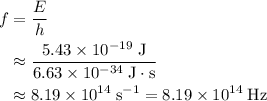 .
.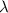 of a photon with a frequency of approximately
of a photon with a frequency of approximately 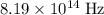 ? The exact answer to that depends on the medium that this photon is travelling through. To be precise, the exact answer depends on the speed of light in that medium:
? The exact answer to that depends on the medium that this photon is travelling through. To be precise, the exact answer depends on the speed of light in that medium: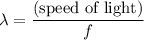 .
.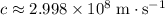 . Therefore, the wavelength of that
. Therefore, the wavelength of that 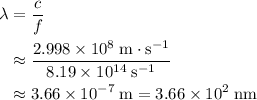 .
.

