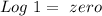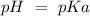Chemistry, 09.07.2020 01:01 jybuccaneers2022
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka of acetic acid is approximately 1. 74 X 10 -5. What is the pH of the resulting solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2


Chemistry, 24.06.2019 02:00
Abase has a. no hydrogen ions. b. few hydrogen ions. c. no hydroxide ions. d. few hydroxide ions.
Answers: 1
You know the right answer?
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka...
Questions




History, 19.04.2020 20:40


Mathematics, 19.04.2020 20:40

Mathematics, 19.04.2020 20:41

Social Studies, 19.04.2020 20:41


Mathematics, 19.04.2020 20:41


Mathematics, 19.04.2020 20:41

English, 19.04.2020 20:41


Chemistry, 19.04.2020 20:41


English, 19.04.2020 20:41

Mathematics, 19.04.2020 20:41

Mathematics, 19.04.2020 20:41

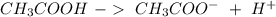
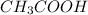 ) and a base (
) and a base (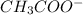 ). Therefore we can write the henderson-hasselbach reaction:
). Therefore we can write the henderson-hasselbach reaction:![pH~=~pKa+Log\frac{[CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0703/3490/99062.png)
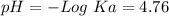
![[CH_3COO^-]=[CH_3COOH]](/tpl/images/0703/3490/ee54c.png)
![\frac{[CH_3COO^-]}{[CH_3COOH]}~=~1](/tpl/images/0703/3490/6e489.png)