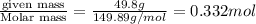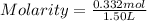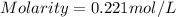Chemistry, 09.07.2020 04:01 applejackjay5818
what is the molarity of a solution that contains 49.8 grams of nai and is dissolved in enough water to make 1.50 liters

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
what is the molarity of a solution that contains 49.8 grams of nai and is dissolved in enough water...
Questions

Mathematics, 11.03.2021 21:40


Mathematics, 11.03.2021 21:40

English, 11.03.2021 21:40

Mathematics, 11.03.2021 21:40





Mathematics, 11.03.2021 21:40


Mathematics, 11.03.2021 21:40


English, 11.03.2021 21:40

Mathematics, 11.03.2021 21:40

Social Studies, 11.03.2021 21:40

Mathematics, 11.03.2021 21:40

Mathematics, 11.03.2021 21:40

Mathematics, 11.03.2021 21:40

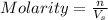
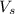 = volume of solution in L
= volume of solution in L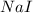 =
=