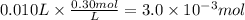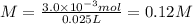Chemistry, 13.07.2020 14:01 praveen35301
If 25 mL of a HCl solution of unknown concentration was neutralized with 10 mL of a 0.30 M NaOH solution, what was the original concentration of the HCl solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
If 25 mL of a HCl solution of unknown concentration was neutralized with 10 mL of a 0.30 M NaOH solu...
Questions

Social Studies, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30

Social Studies, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30

Biology, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30


Mathematics, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30





Mathematics, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30

Mathematics, 19.08.2019 13:30



History, 19.08.2019 13:30