Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
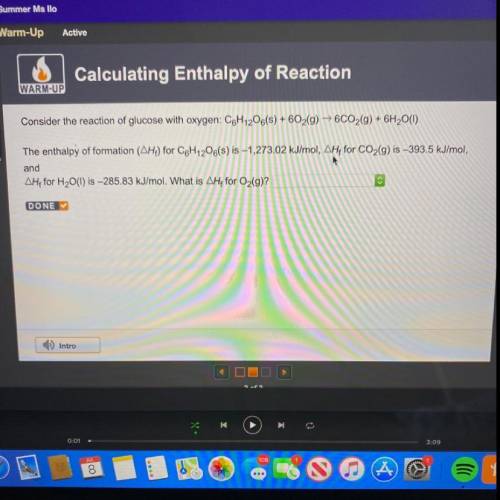
Consider the reaction of glucose with oxygen: C6H12O6(s) + 602(g) → 6CO2(g) + 6H2O(1)
The enthalpy...
Questions

Mathematics, 27.03.2021 01:00


Mathematics, 27.03.2021 01:00




Mathematics, 27.03.2021 01:00

Mathematics, 27.03.2021 01:00


Mathematics, 27.03.2021 01:00

Social Studies, 27.03.2021 01:00


Chemistry, 27.03.2021 01:00

Mathematics, 27.03.2021 01:00




Mathematics, 27.03.2021 01:00


Mathematics, 27.03.2021 01:00