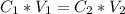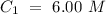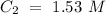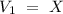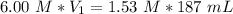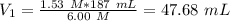Chemistry, 13.07.2020 17:01 bbombard21
What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be diluted to 187 mL to make a 1.53 M solution of sodium hydroxide

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be diluted to 187 mL t...
Questions

History, 01.10.2019 14:50


Biology, 01.10.2019 14:50

Mathematics, 01.10.2019 14:50





Mathematics, 01.10.2019 14:50

English, 01.10.2019 14:50


Geography, 01.10.2019 14:50



History, 01.10.2019 14:50



Business, 01.10.2019 14:50


History, 01.10.2019 14:50