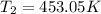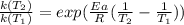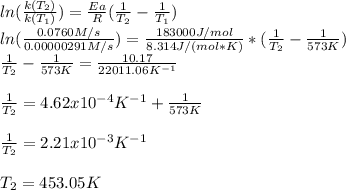Chemistry, 13.07.2020 19:01 nsutton9985
The activation energy for the decomposition of HI is 183 kJ/mol. At 573 K, the rate constant was measured to be 2.91 x 10^{-6} M/s. At what temperature in Kelvin does the reaction have a rate constant of 0.0760 M/s

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
The activation energy for the decomposition of HI is 183 kJ/mol. At 573 K, the rate constant was mea...
Questions

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

History, 11.09.2020 22:01

Spanish, 11.09.2020 22:01

German, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Chemistry, 11.09.2020 22:01

Social Studies, 11.09.2020 22:01

English, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Chemistry, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Biology, 11.09.2020 22:01

History, 11.09.2020 22:01